Sayer Ji
Nov 23, 2025 ∙ Paid
Read and share the X post dedicated to this article: https://x.com/sayerjigmi/status/1992577130874450058?s=20
What if the foundational premise of modern medicine is incomplete?
What if you’re not primarily being “infected” by viruses, but rather responding to chemical exposures?
And what if your body’s healing response—the one we call “disease”—is actually being misinterpreted?
Let me show you the evidence.
Introduction: A Mystery at the Heart of Modern Medicine
Imagine this: You take a common over-the-counter painkiller for a headache. Your liver suffers damage from the drug—but here’s the puzzle: the medication never physically reaches your heart, your lungs, or your brain. Yet hours later, your heart rate becomes irregular, your breathing grows shallow, and fever wraps around you like a shroud. How did the drug’s damage confined to your liver become a whole-body crisis?
The conventional textbook answer is simple: “The drug circulates in your bloodstream and affects multiple organs.” But the real story—revealed by cutting-edge molecular biology—is far more unsettling and illuminating. Your damaged liver cells aren’t just passively leaking toxins into your circulation. They’re actively packaging the molecular fingerprints of their injury—oxidative stress molecules, activated death signals, inflammatory chemicals—into nano-sized vesicles and sending them throughout your body like biological text messages of distress. These tiny packages then infect healthy cells with the same injury program that’s destroying your liver. It’s cellular contagion without any virus involved.
This process—cells broadcasting distress signals to distant tissues—is not an isolated phenomenon or a theoretical quirk. It happens with pesticide exposure, heavy metal poisoning, air pollution, pharmaceutical side effects, and countless other environmental toxins. And here’s what makes this discovery deeply, personally relevant: these same cellular distress signals can mimic infectious disease so perfectly that they’ve fooled physicians, epidemiologists, and public health officials for centuries.
The Case That Changed Everything: EVALI
In 2019, the United States experienced an unexpected crisis. Over 2,800 young Americans, mostly teenagers and young adults, were hospitalized with what appeared to be a novel, aggressive viral pneumonia. Doctors prescribed antibiotics. Some patients spent weeks on mechanical ventilators, fighting for each breath. The outbreak spread from state to state, creating panic reminiscent of the emergence of a dangerous new pathogen. Fear rippled through social media: Was a new virus loose in the population?
The clinical picture fit the pattern of infection perfectly. Patients presented with fever, cough, dyspnea, and bilateral lung infiltrates on chest imaging—findings that radiologists and infectious disease specialists interpreted as evidence of severe viral or atypical bacterial pneumonia.1 Yet when the Centers for Disease Control and Prevention investigated, they uncovered a truth that overturned the initial assumption: no virus was involved. The culprit was vitamin E acetate, a chemical adulterant added to illicit cannabis vape cartridges. Vitamin E acetate, when inhaled repeatedly, severely damages lung tissue and triggers intense inflammatory responses—mimicking infection so closely that it fooled experienced clinicians.
The CDC confirmed vitamin E acetate in bronchoalveolar fluid from 48 of 51 EVALI patients sampled, versus zero in healthy controls.2 It was a toxin, not a microbe. Yet the sick people shared a common exposure source—the vape cartridges circulating through social networks—which created the appearance of person-to-person transmission. Entire friend groups fell ill together. Families seemed to pass the illness along. All of this happened without any contagious virus being involved.
The EVALI case is modern. But this pattern is ancient.
An Ancient Mystery Revisited
In medieval Europe, periodic waves of a horrifying affliction swept through communities. Victims suffered burning pain so severe they begged for amputation, watched their limbs turn gangrenous and black, endured convulsions and hallucinations, and frequently died. Entire villages fell ill within days. Because of this clustering pattern, physicians and clergy were convinced they were witnessing a contagious plague. They called it St. Anthony’s Fire, and they prayed for divine intervention because medicine seemed powerless to stop it.3
It took centuries to understand the truth: St. Anthony’s Fire was not contagious at all. It was ergotism—poisoning by toxic alkaloids produced by a fungus (Claviceps purpurea) that contaminated rye grain during damp growing seasons. Entire communities fell ill simultaneously not because the disease spread person-to-person, but because everyone in the village ate the same tainted harvest at the same time.4 Only in 1695 was the true cause identified. For centuries, ergotism was misdiagnosed as plague.
This raises an unsettling question: How many historical outbreaks that we’ve attributed to viruses or bacteria actually resulted from shared toxic exposures? And more immediately relevant: What does this pattern tell us about how we diagnose, treat, and prevent disease today?
The Scientific Breakthrough: Understanding Extracellular Vesicles
To answer these questions, we need to understand a molecular mechanism that has only recently come into scientific focus: extracellular vesicles (EVs)—nano-sized packets that cells release under stress. Recent peer-reviewed research has begun to explain how chemical exposures can spread illness through a population in patterns that resemble contagion, why symptoms of chemical poisoning often mimic infection, and why so many modern diseases might be rooted in environmental toxins rather than microbes.
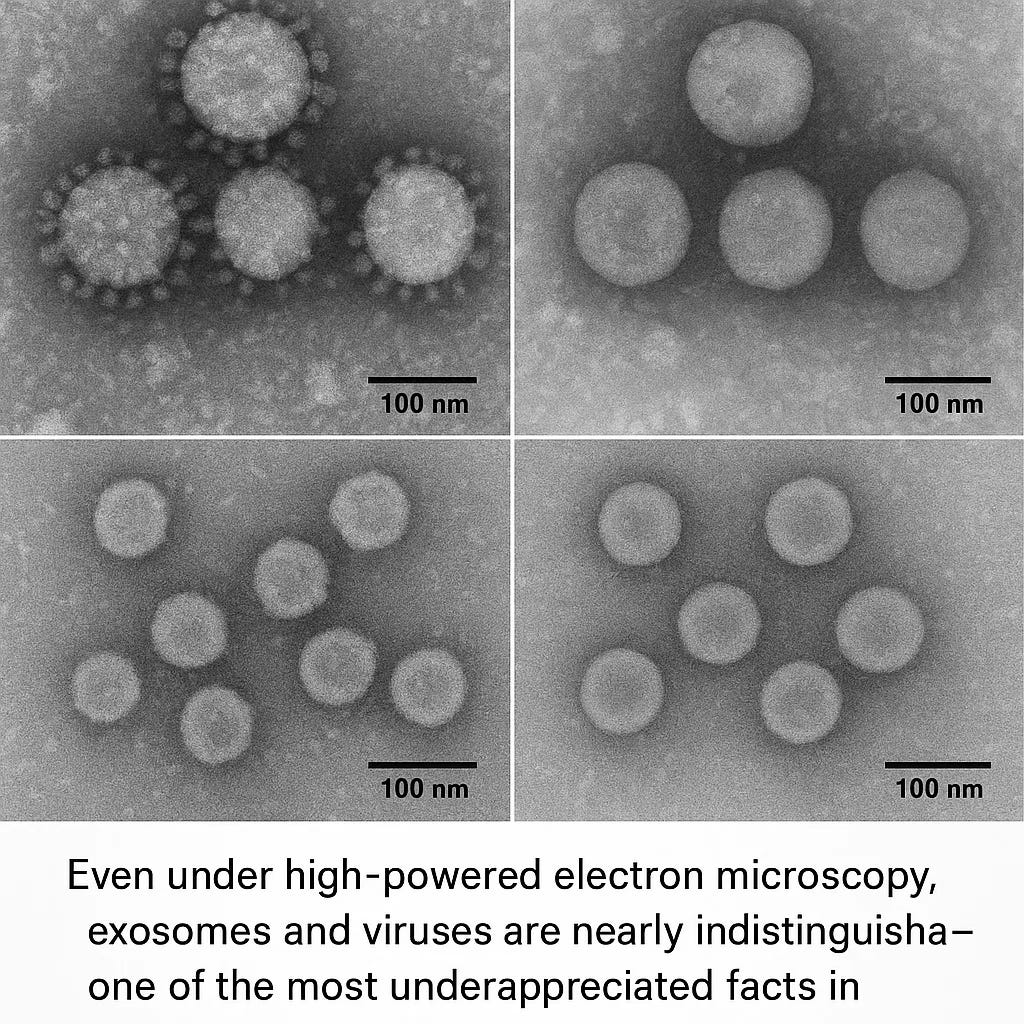
Here’s what makes EVs particularly fascinating—and troubling for our medical paradigm: they are so similar to viruses in size, structure, and function that laboratory scientists struggle to tell them apart. An exosome (a type of EV) is roughly 30–150 nanometers in diameter. HIV particles measure about 120 nanometers. Coronaviruses range from 80–120 nanometers. Under an electron microscope, a trained virologist might not be able to definitively say which is which. Yet these tiny packets—whether we call them exosomes or viruses—can trigger the same cellular responses, the same immune activation, the same constellation of symptoms we call illness.
This review synthesizes that research. We’ll examine landmark studies showing how chemical exposures—from pharmaceutical drugs to pesticides, heavy metals, and air pollutants—trigger cells to release virus-like particles. We’ll trace how these particles propagate distress signals systemically, creating whole-body illness patterns that mimic infection. We’ll reexamine historical disease outbreaks through this new lens. We’ll explore why exosomes and viruses are essentially indistinguishable at the molecular level. And we’ll introduce a controversial but increasingly empirically-grounded framework—the Xenogen Hypothesis—suggesting that what we’ve called “viruses” might often represent the body’s own adaptive response to environmental stress.
Understanding this distinction—and the science behind it—could fundamentally reshape how we think about health, immunity, prevention, and the nature of disease itself.
Extracellular Vesicles: The Hidden Language Your Cells Speak
What They Are and How They Work
Extracellular vesicles are tiny, membrane-bound packets released by virtually every cell in your body.5 Think of them as biological envelopes—each one a small sac containing proteins, lipids, messenger RNAs, microRNAs, and other molecular cargo that reflects the state of the cell that released it.
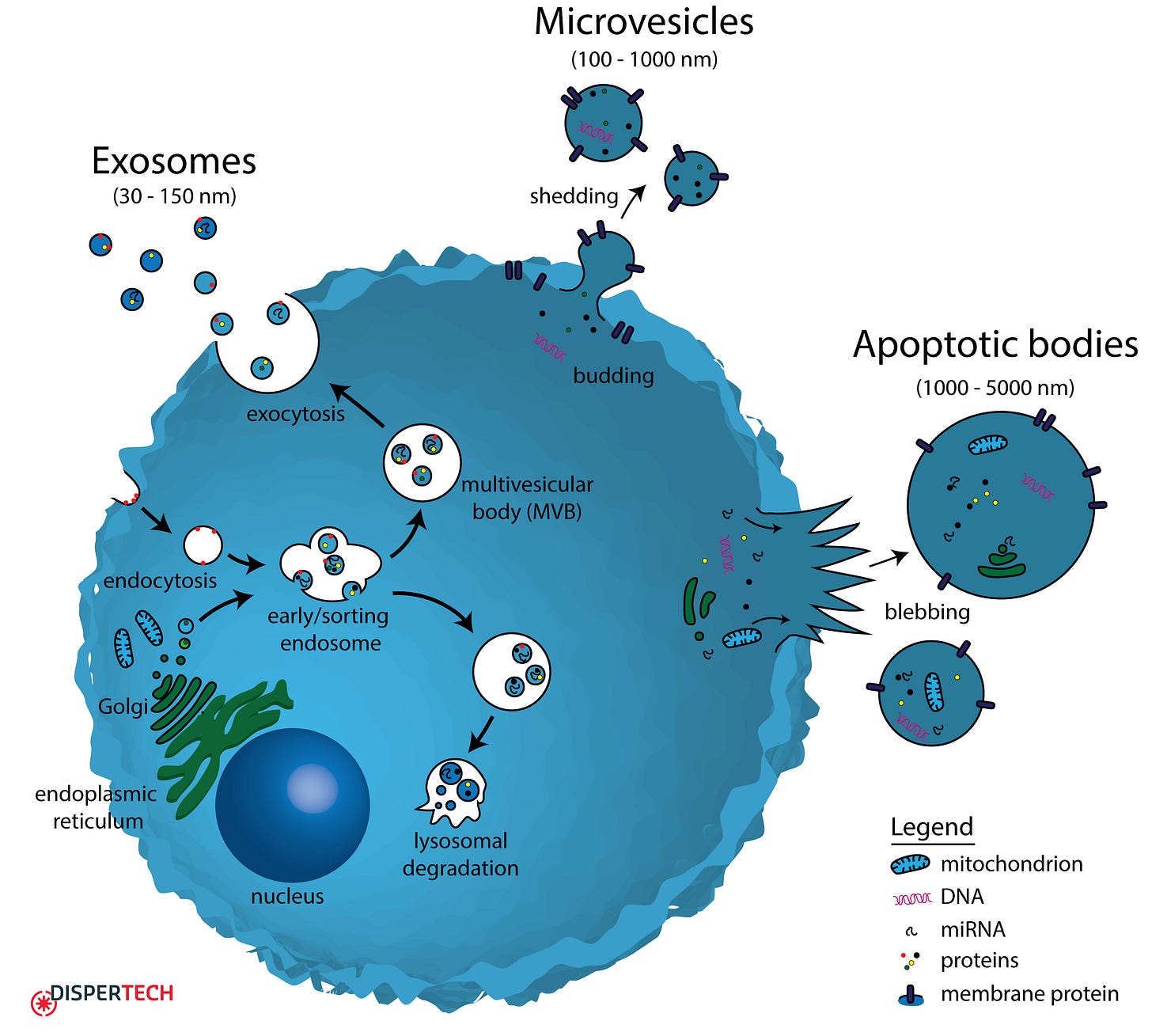
EVs come in several types. Exosomes form inside cells in specialized compartments called multivesicular bodies; when these structures fuse with the cell membrane, exosomes spill into the extracellular space. Microvesicles bud directly from the cell surface, like tiny bubbles pinching off. Apoptotic bodies are released by cells undergoing programmed death, like cellular emergency distress signals. Despite their different origins, all EVs serve a similar fundamental function: they carry information from one cell to another.6
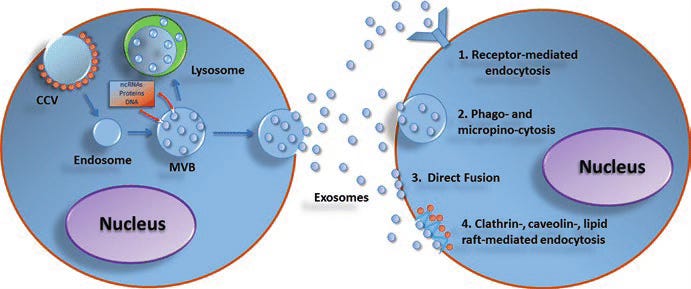
Under normal circumstances, this intercellular communication is actually beneficial. Your immune cells use exosomes to coordinate responses. Stem cells release healing vesicles that reduce inflammation. Your gut microbiota package protective molecules into exosomes that enter your bloodstream and fortify your intestinal barrier. EVs are, in essence, the language through which your body’s cells speak to one another about health and threat.
The Stress Response: When Toxins Turn Cells Into Broadcasters
But here’s where things become concerning: when your cells encounter environmental stressors—pharmaceutical drugs, pesticides, heavy metals, air pollutants, electromagnetic fields—they dramatically increase the release of EVs.7 It’s as though a cell under attack sends out an urgent cellular alarm. But instead of just calling for help, the cell packages the molecular signature of its injury—the oxidative stress molecules, the activated death proteins, the inflammatory chemicals—and ships these distress signals throughout your body.
This is where the mechanism becomes both remarkable and troubling. Recipient cells that take up these stress-laden vesicles often respond as though they themselves had been directly exposed to the toxin. They activate the same injury programs. They suffer the same damage. The injury spreads—not through the bloodstream carrying more toxin, but through vesicles carrying the signal of injury.
The Evidence: When Drugs and Toxins Trigger Viral-Like Signals
The Acetaminophen Story: A Watershed Moment in Understanding Toxicity
In 2018, a pivotal study published in Scientific Reports provided dramatic proof of this mechanism. Researchers gave mice toxic doses of acetaminophen (the active ingredient in Tylenol).8 As expected, the mice’s livers suffered severe damage. But here’s where the experiment became revelatory: the scientists isolated exosomes from the damaged mice’s blood and transferred them to healthy mice that had never been exposed to acetaminophen.
The result was striking. The recipient mice—which had never touched acetaminophen—developed liver damage. Their hepatocytes (liver cells) began dying in the same pattern. Their bodies released the same inflammatory cytokines (TNF-α and IL-1β) that had flooded the original poisoned mice. Their livers activated the same apoptotic death cascade: phosphorylated JNK, pro-apoptotic Bax, cleaved caspase-3.9 In essence, the exosomes carried the injury from one mouse to another.
Let this sink in: one liver cell exposed to a drug can package its distress into an exosome, release it into the bloodstream, and hours later, that exosome can enter a distant cell—in the heart, the kidney, the brain—and cause that cell to execute the same death program. The toxin itself never reaches these distant cells. Only the message of injury does. This is how a localized drug effect becomes a systemic crisis.
Reframing Viral Mechanisms: Exosomes, Toxicity, and the Xenogen Hypothesis
In a 2018 study published in Scientific Reports, researchers explored the effects of acetaminophen-induced liver injury in mice, with an initial focus on validating toxicological mechanisms of liver damage. However, their findings inadvertently cast light on a much deeper—and potentially paradigm-shifting—insight into the nature of so-called "viral" dis…
A Pattern Across Toxins: Pesticides, Metals, and More
The acetaminophen study is not an anomaly. Recent research demonstrates that this EV-mediated injury signaling occurs across a spectrum of chemical exposures. When neurons are exposed to rotenone (a pesticide), they release exosomes laden with specific microRNAs that cause mitochondrial dysfunction in naive neurons.10 When brain immune cells (microglia) encounter paraquat (an herbicide), they release inflammatory exosomes that accelerate neurodegeneration in dopamine neurons—a mechanism that may underlie some cases of Parkinson’s disease.11 When muscle tissue is exposed to arsenic, EV-mediated signaling disrupts the normal regenerative response to injury.12
The pattern is consistent: toxins don’t just damage the cells they directly contact. They transform those damaged cells into broadcasters of injury signals. Those signals, packaged in virus-sized vesicles, propagate to other tissues and organs. Multiple organs can suffer dysfunction without ever being directly exposed to the original toxin.
Summary Table: How Different Toxins Trigger EV-Mediated Injury Signals
When Toxins Masquerade as Epidemics: The Pattern of Pseudo-Contagion
If toxin-induced EVs can trigger injury signals that propagate through multiple tissues and people, a provocative question emerges: could disease outbreaks historically attributed to infectious agents actually represent mass toxic exposures, with EV-mediated signaling creating the illusion of contagion?
Medieval Europe: The Mystery of St. Anthony’s Fire
From roughly the 10th century through the 17th century, periodic waves of St. Anthony’s Fire devastated medieval communities. The affliction produced horrifying symptoms: a burning sensation in the limbs so intense that victims begged for amputation, gangrene spreading through fingers and toes, convulsions, hallucinations, and frequently death.13
Because entire villages fell ill over the course of days or weeks, physicians and religious authorities logically concluded that an infectious plague was spreading from person to person. They prayed, they bled patients, they searched for natural causes. Yet in their overwhelming majority, patients shared a single exposure: they had all consumed grain from the same local harvest. What they didn’t know was that the grain was contaminated with ergot—a fungal pathogen that produces ergotamine, lysergic acid, and other toxic alkaloids.
Ergotism is not contagious between humans. Yet because entire communities consumed tainted grain simultaneously, everyone suffered illness in a synchronized pattern. To contemporary observers, it appeared as an infectious epidemic. It took nearly 700 years for scientists to identify ergot as the culprit.14
Now consider this through the lens of EV biology: the ergot alkaloids triggered massive oxidative stress in exposed individuals’ tissues. Their damaged cells released storms of stress-laden exosomes. These vesicles propagated inflammatory and apoptotic signals throughout each victim’s body, amplifying systemic effects. But because the primary exposure (contaminated grain) was shared among community members, the illness appeared to follow an infection-like temporal pattern—everyone got sick around the same time. For 700 years, this temporal clustering fooled the medical establishment into believing contagion was at play.
2019: The EVALI Crisis—A Modern Parallel
The pattern repeated almost perfectly in 2019. What made EVALI remarkable was not just that a toxin mimicked infection, but that it happened in the age of modern medicine—yet still initially fooled experts. Over 2,800 young Americans required hospitalization. Doctors ordered antibiotics and antivirals. Infectious disease specialists debated which pathogen was responsible. State health departments issued alerts. Social media exploded with theories about a new virus.
Then the CDC traced the common exposure: vitamin E acetate in illicit vape cartridges. Vitamin E acetate, when inhaled, devastates lung tissue and triggers a severe inflammatory response that clinically and radiographically mimics viral pneumonia. The CDC confirmed vitamin E acetate in bronchoalveolar fluid from 48 of 51 patients tested, but in zero healthy controls.15
EVALI demonstrates three critical points: (1) Toxic injury can produce clinical and radiographic findings that are indistinguishable from infection, (2) When multiple individuals share exposure to the same toxin source, the temporal clustering of illness creates the appearance of epidemic spread, and (3) Even in the 21st century, with modern diagnostic tools, we can misattribute toxic illness to infectious causes.
At the cellular level, the EVALI victims’ damaged lungs likely released massive quantities of inflammatory exosomes—vesicles loaded with danger signals and inflammatory microRNAs. These exosomes propagated distress signals not just locally in the lungs, but systemically, triggering fever, malaise, and systemic inflammation. Patients appeared genuinely infected. Clinically, they were infected—but with toxic signals, not with viruses.
The Diagnostic Problem: Why We Confuse Exosomes and Viruses
A critical factor enabling this confusion—between toxin-induced illness and viral infection—is the remarkable biophysical and molecular similarity between extracellular vesicles and viruses. They are so alike that laboratory scientists, equipped with sophisticated equipment, struggle to reliably distinguish them.
Size, Shape, and Density: Nature’s Convergent Design
Exosomes measure 30–150 nanometers in diameter. HIV particles measure approximately 120 nanometers. Coronaviruses (SARS-CoV-2, MERS, and relatives) measure 80–120 nanometers. These particles are so similar in size that when a laboratory technician performs density gradient ultracentrifugation—a classical method for purifying viruses—exosomes and viruses pellet out in the same fraction.16
Both are roughly spherical. Both have a lipid bilayer membrane enclosing a core of nucleic acids and proteins. Both are small enough to evade some immune responses while remaining large enough to package substantial molecular cargo. When a virologist peers at them under electron microscopy, both appear as round or slightly pleomorphic vesicles. Without specific antibody labeling or other biochemical markers, an electron micrograph cannot reliably distinguish between them.
As one comprehensive virology review stated: “the fact that exosomes are similar in size, shape, and density to many viruses makes separating exosomes from viruses a veritable challenge.”
Molecular Markers: The Blurry Boundary Between “Self” and “Other”
The molecular overlap may be even more troubling than the functional similarities suggest. Recall the 2015 study “Conserved and host-specific features of influenza virion architecture” that first fully characterized what influenza viruses are actually composed of. The researchers found that virions contain substantial quantities of host proteins as well as viral proteins—influenza virions produced by mammalian and avian hosts have distinctly different protein compositions depending on their origin. The virus is as much comprised of biological material from the host as it is of viral genetic material. I dive deeper into this in my article: Why Everything You Learned About Viruses is WRONG.
This finding becomes even more unsettling when we examine the molecular markers themselves. Enveloped viruses acquire their membrane by budding from host cells, incorporating host-derived proteins into their viral envelope. One canonical exosomal marker protein is CD63, a tetraspanin. This same CD63 protein is found on HIV particles and hepatitis B virus particles. Viruses and exosomes don’t just look alike—they share molecular markers.17
This is precisely why we face a contagion illusion. If the proteins that define a virus are identical to the proteins that define a host cell product, what exactly are we identifying when we say we’ve detected a virus? We’re identifying a protein signature—not necessarily a pathogenic entity.
Conversely, exosomes released from virus-infected cells contain viral nucleic acids and viral proteins. These exosomes can transfer complete viral genomes to new cells, which then begin producing infectious virus—without any traditional free virion involvement.18 In the case of hepatitis C, infected liver cells release exosomes containing the complete HCV RNA genome. When these exosomes encounter new hepatocytes, they fuse with them, and the recipient cells begin producing infectious HCV.
At what point does an exosome become a virus? The answer is: there may be no clear boundary. Nature appears to exploit a biological continuum, with viruses having co-opted or evolved alongside exosomal pathways. From an extracellular vesicle perspective, viruses are simply exosomes that happen to carry highly organized genetic instructions for replication. From a virology perspective, exosomes are simply vesicles that cells use, and viruses have learned to use these same pathways.
Diagnostic Consequences: When Exosomes Masquerade as Viruses
The theoretical indistinguishability between exosomes and viruses became catastrophically practical during the COVID-19 pandemic. A 2024 peer-reviewed study examining the role of exosomes in PCR testing found that “PCR tests have zero specificity in vivo due to the exosome RNA.” The researchers concluded that the RNA code detected in COVID-19 PCR tests—previously attributed to SARS-CoV-2—actually belonged to the respiratory-virus-induced immune system response by human cells liberating exosomes.
The implications are staggering: the study found that the second and subsequent COVID-19 outbreak waves reported globally were largely artifacts of false-positive results. This explains not only the peculiar patterns of “waves” but also the persistent failure of vaccines to prevent infection and reinfection—we were never precisely identifying what we were vaccinating against in the first place.
This is the contagion illusion made manifest. When we cannot distinguish at the molecular level between a virus and an exosome, between a genuine pathogenic entity and the body’s own immune signaling mechanism, our diagnostic architecture collapses. We are left detecting signals, not causes. Indeed, we dived deeper into this topic in the article PCR Testing Unmasked: The Illusion of Viral Detection in a Sea of Nucleic Acids.
Diagnostic Pitfalls and Clinical Implications
These overlaps create concrete diagnostic problems in clinical practice. PCR tests designed to detect viral nucleic acids may amplify genetic sequences present in exosomal cargo—defective viral genomes, retrotransposon sequences, or viral-derived microRNAs packaged in EVs—even when no replication-competent virus is present. Antibody-based tests may register false positives if exosomes express viral antigens acquired from infected donor cells. Electron micrographs of suspected viral particles may actually show exosomes.
These diagnostic challenges were particularly acute during the early phases of the COVID-19 pandemic. Some skeptics raised the legitimate technical question of whether electron micrographs purporting to show SARS-CoV-2 virions might instead show exosomes. While the scientific consensus unequivocally identifies SARS-CoV-2 as a real, infectious agent, the legitimacy of the morphological question highlights how difficult it truly is to distinguish these particles.
The practical implication is that clinicians and researchers must employ a cautious, multimodal approach to diagnosis. Relying on a single laboratory method—electron microscopy, PCR, or antibody assays—is inadequate when exosomes and viruses may be present simultaneously or when EV contamination is possible. This recognition doesn’t cast doubt on virology itself, but it suggests that diagnostic protocols require refinement and cross-validation, particularly in novel outbreaks where the etiological agent is initially uncertain.





No hay comentarios:
Publicar un comentario